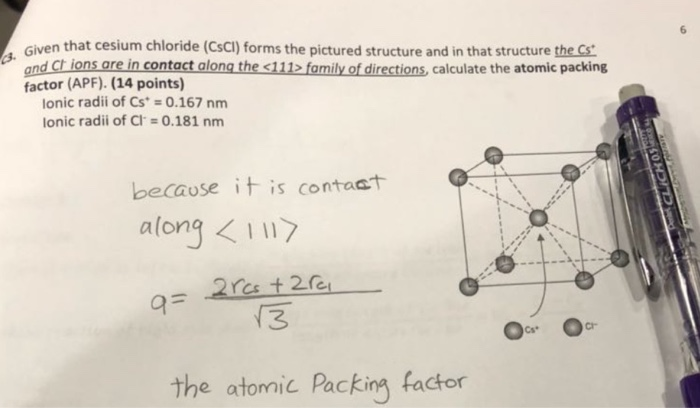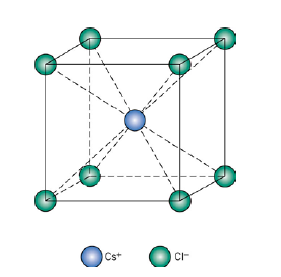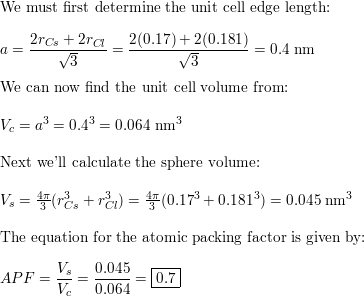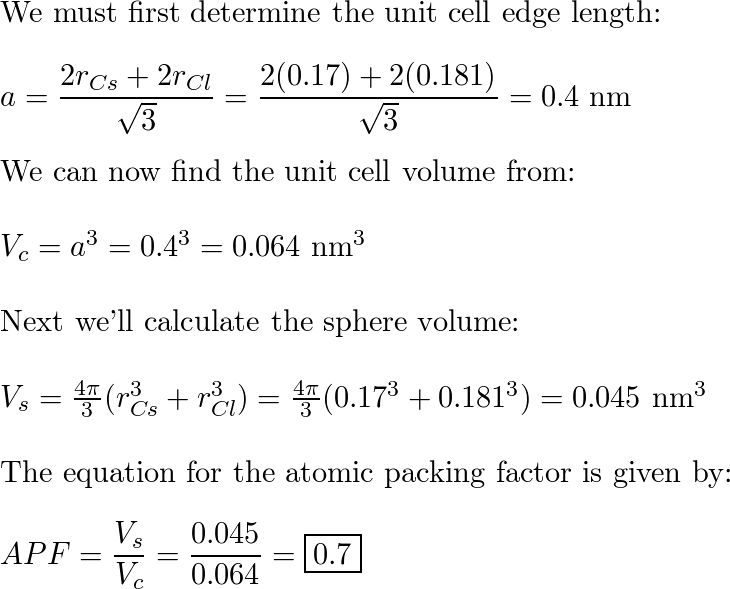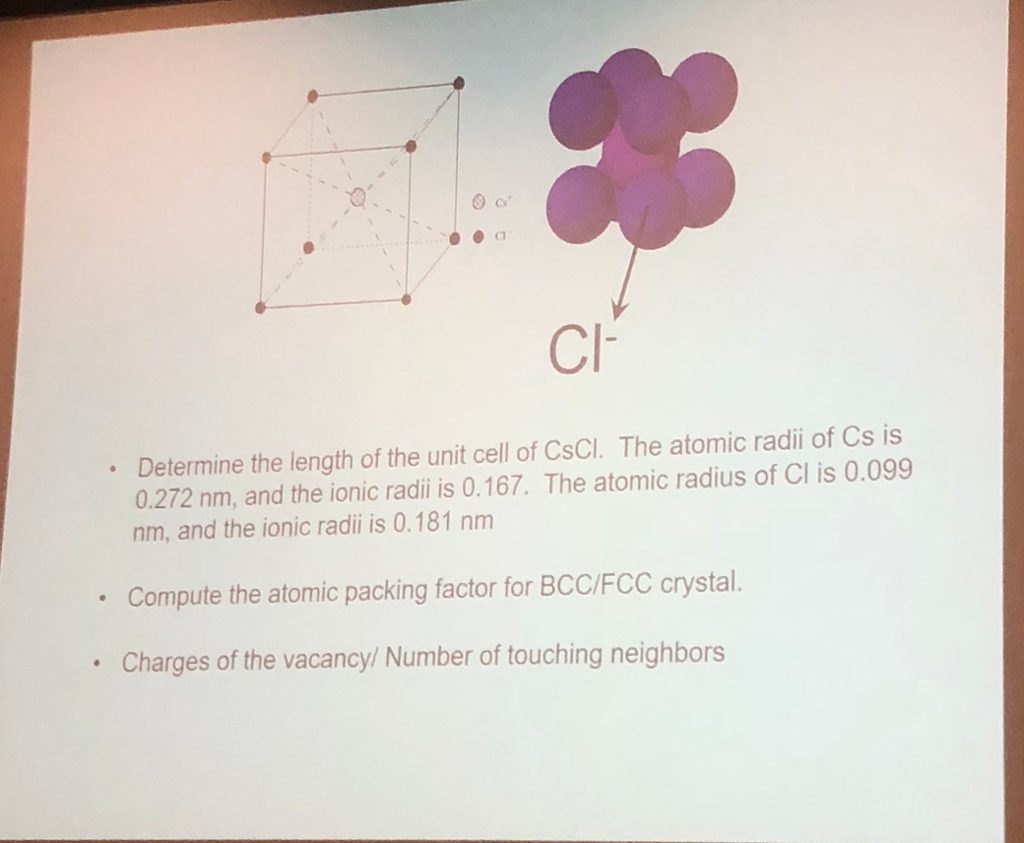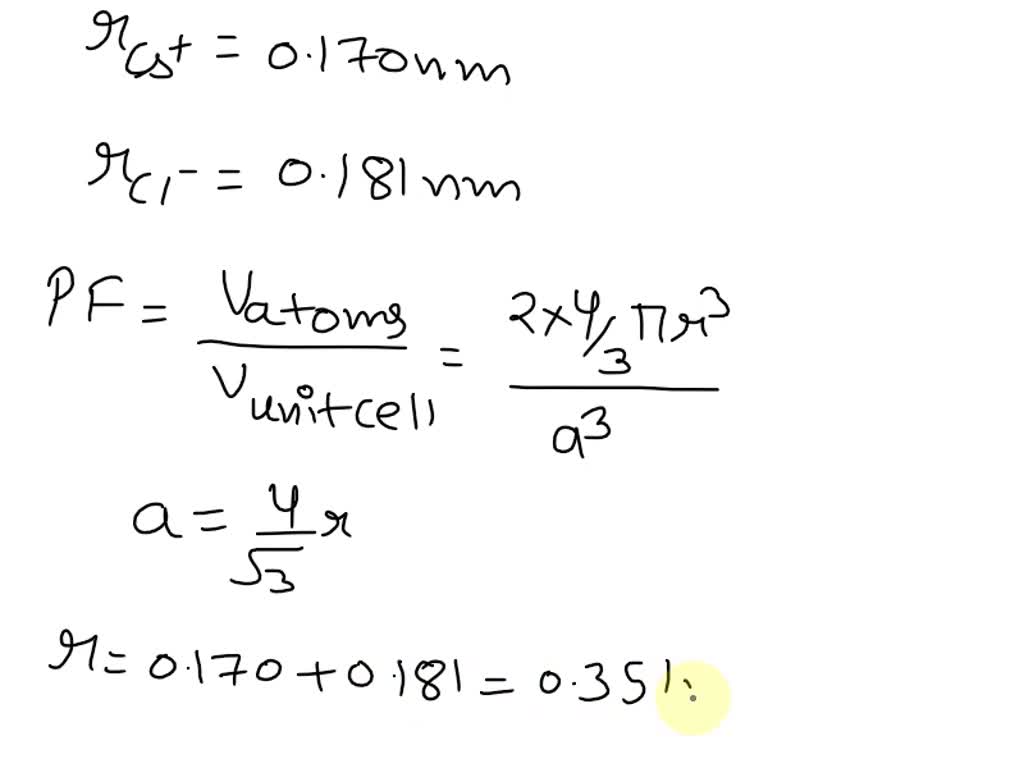
SOLVED: 'Compute the atomic packing factor for cesium chloride assuming that the ions touch along the cube diagonals. Ionic radii of Cs* and Cl" are 0.170 nm and 0.181 nm, respectively.'
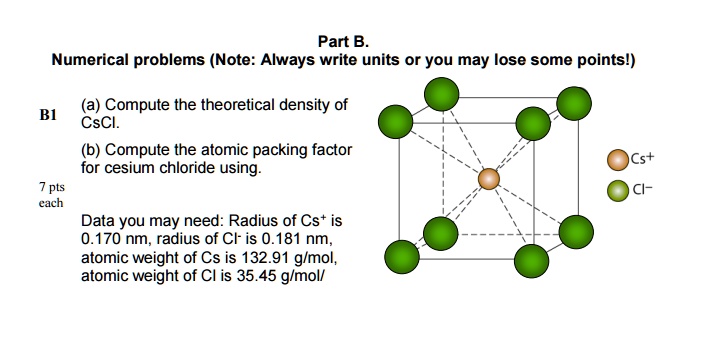
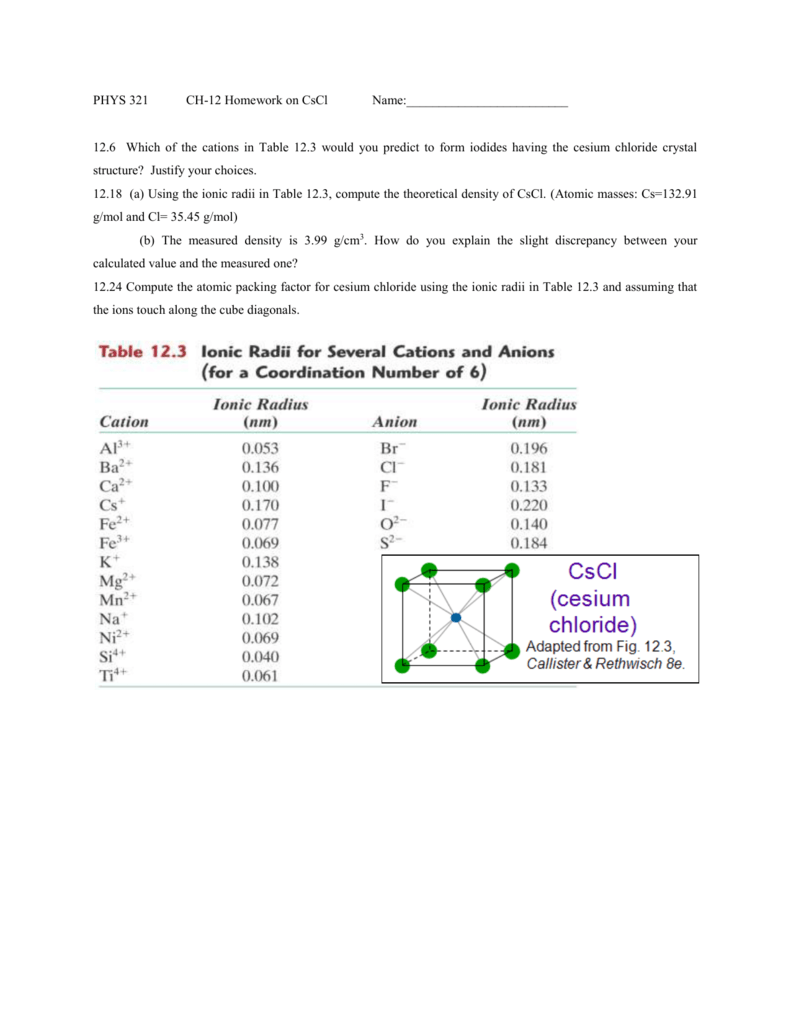
SOLVED: Numerical problems (Note: Always write units or you may lose some points!) (a) Compute the theoretical density of CsCl. (b) Compute the atomic packing factor for cesium chloride using the following

crystals - What did Feynman say about cesium chloride and body-centered cubic structure? - Physics Stack Exchange
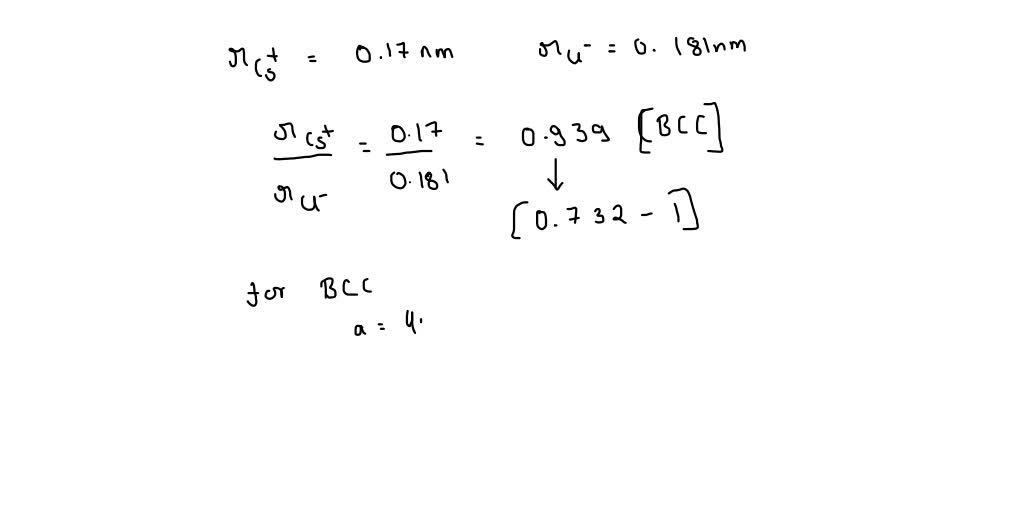
SOLVED: Compute the atomic packing factor for cesium chloride assuming that the ions touch along the cube diagonals. Ionic radii of Cs+ and Cl- are 0.170 nm and 0.181 nm, respectively.
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering

ISSUES TO ADDRESS... How do atoms assemble into solid structures? (for now, focus on metals) How does the density of a material depend on its structure? - ppt download