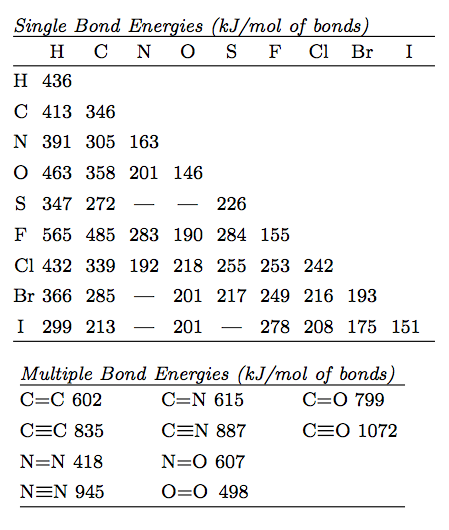
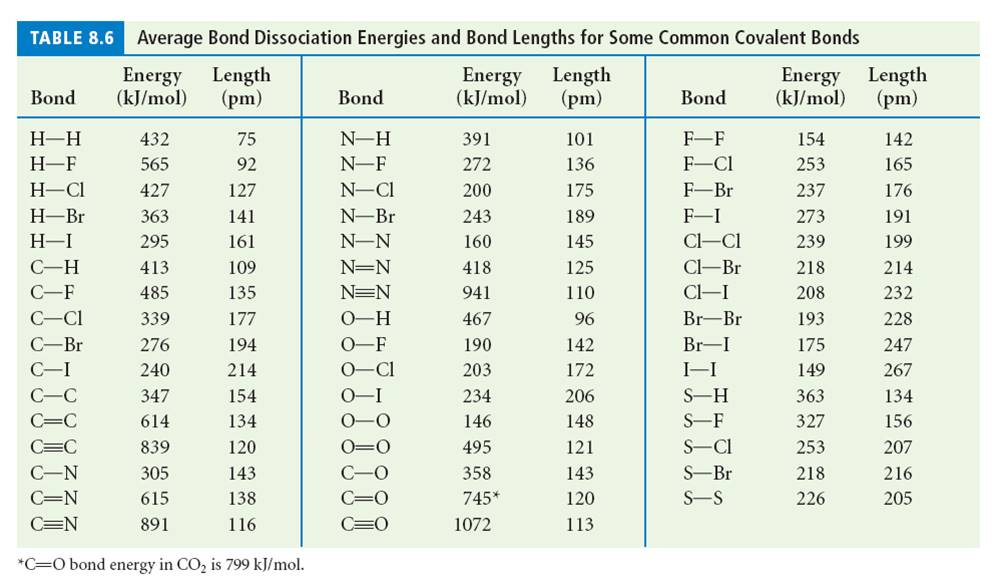
Using the bond energies in Table 7.2, determine the approximate enthalpy change for each of the following reactions: (a) Cl 2 ( g ) + 3 F 2 ( g ) →

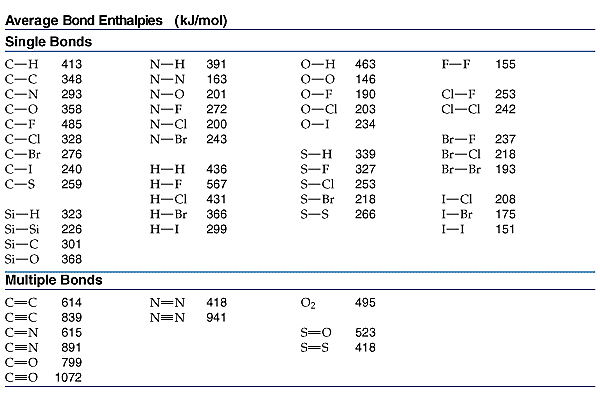
SOLVED: Calculate the bond energy for the formation of boron trifluoride: L Average Bond Enthalpies (kJ/mol) Single Bonds: C-H 413 391 348 163 293 201 358 272 485 200 328 243 463
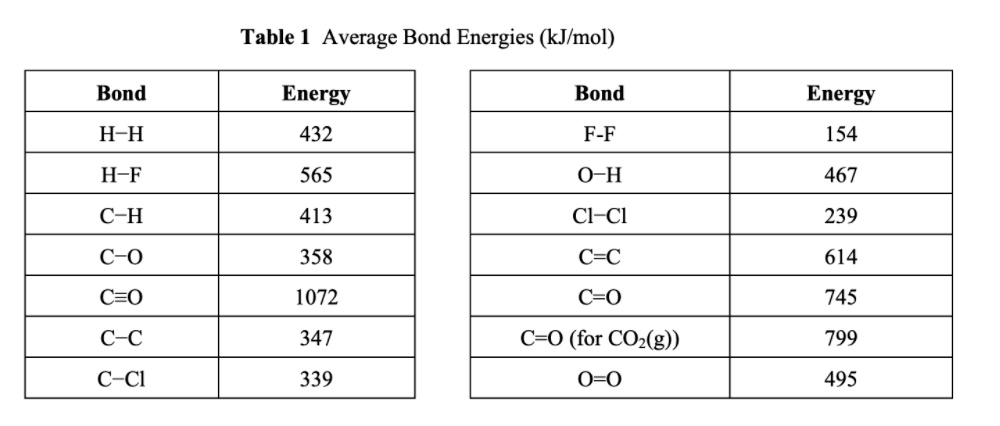
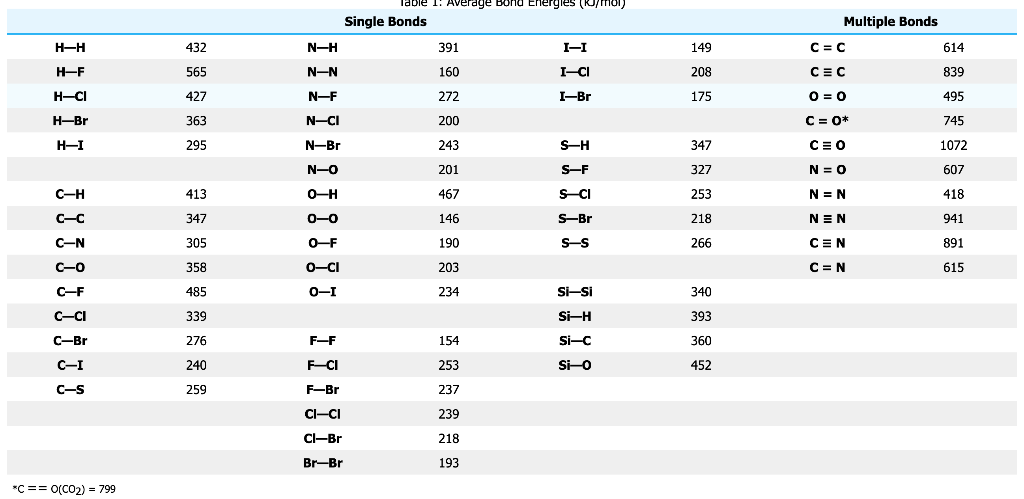
SOLVED: Table 1: Average Bond Energies (kJ/mol) Bond Energy H-H 432 F-F 154 H-F 565 O-H 467 C-H 413 Cl-Cl 239 C-O 358 C-C 614 CO 1072 C-O 745 C-C 347 C-O (for CO2(g)) 799 C-Cl 339 O-O 495

Table 11 from Theoretical studies of transition-metal hydrides. 1. Bond energies for MH+ with M = Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn | Semantic Scholar
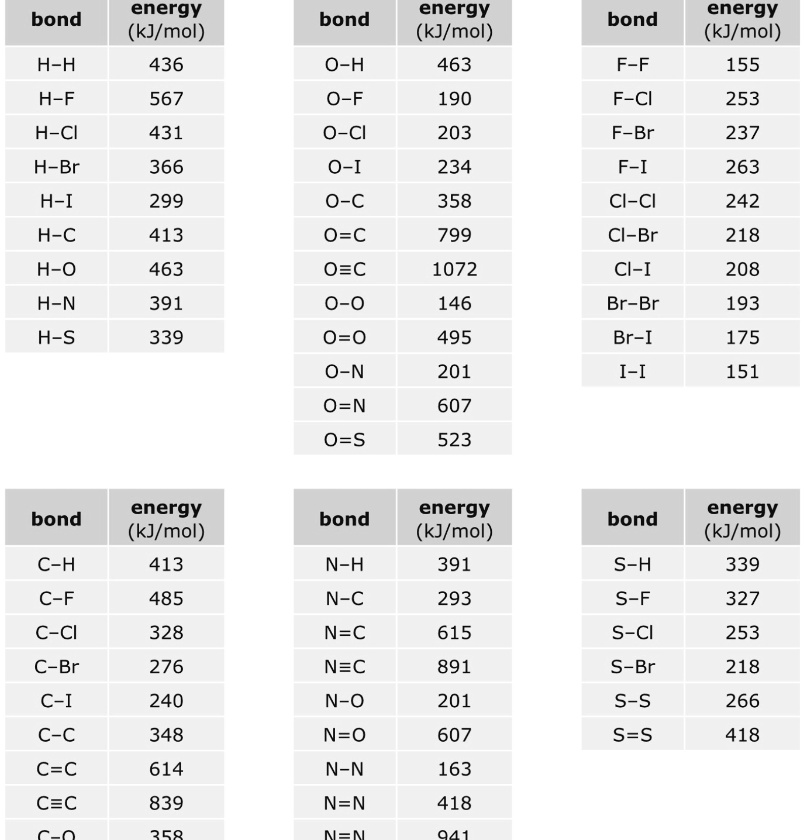
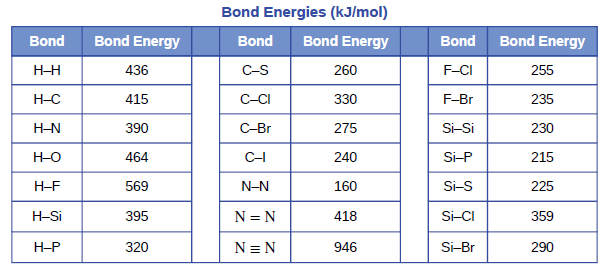
SOLVED: Bond Energy (kJ/mol): 436, 567 Bond Energy (kJ/mol): 463 Bond Energy (kJ/mol): 155, 253, 237 H-H O-H F-F H-F O-F 190 F-Cl H-Cl 431 O-Cl 203 F-Br H-Br 366, 299 O-I


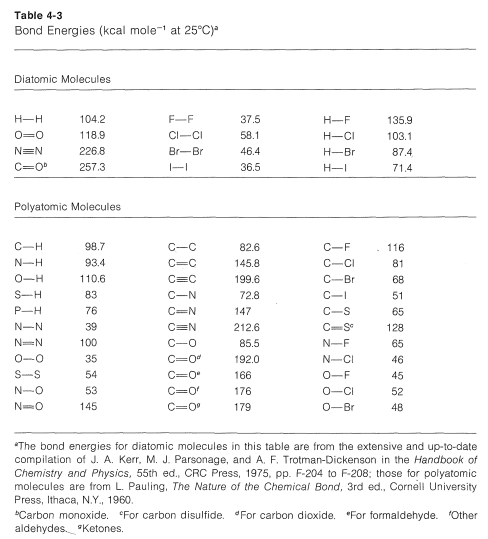


![PDF] Bond dissociation energies | Semantic Scholar PDF] Bond dissociation energies | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ce7c065d005590afbab6bb30de466e75e50d4f86/7-Table3-1.png)